Family owned & operated since 1973, Martin Water Conditioning has been providing homes and families with safe, clean water utilizing Kinetico’s advanced home water systems.
As an Authorized, Independent Kinetico dealer, Martin Water provides on-site testing to evaluate the quality of water coming into your home, determining the whole house water system that is right for your family’s needs.




– Removes more contaminants than any other reverse osmosis.
– Rated Consumers Digest Best Buy.
– Extremely fast flow from the faucet
– Smartly designed to be convenient and economical.
Watch Video – Click Here
Read What Others Are Saying – Get a Quick Quote


For more than 50 years, Kinetico has taken a smarter approach to solving water problems. These systems treat water more efficiently, effectively, and economically. With more contaminant removal and no spikes in performance, you can be confident they will outperform other options available and provide you with clean, clear water for years and years. It’s why so many people around the world rely on our products. With Kinetico, every time you turn on your tap, you know you’re getting the best water and only water.
Brilliantly engineered to be simply the most efficient and effective water softeners, drinking water systems and specialty treatment systems in the world.
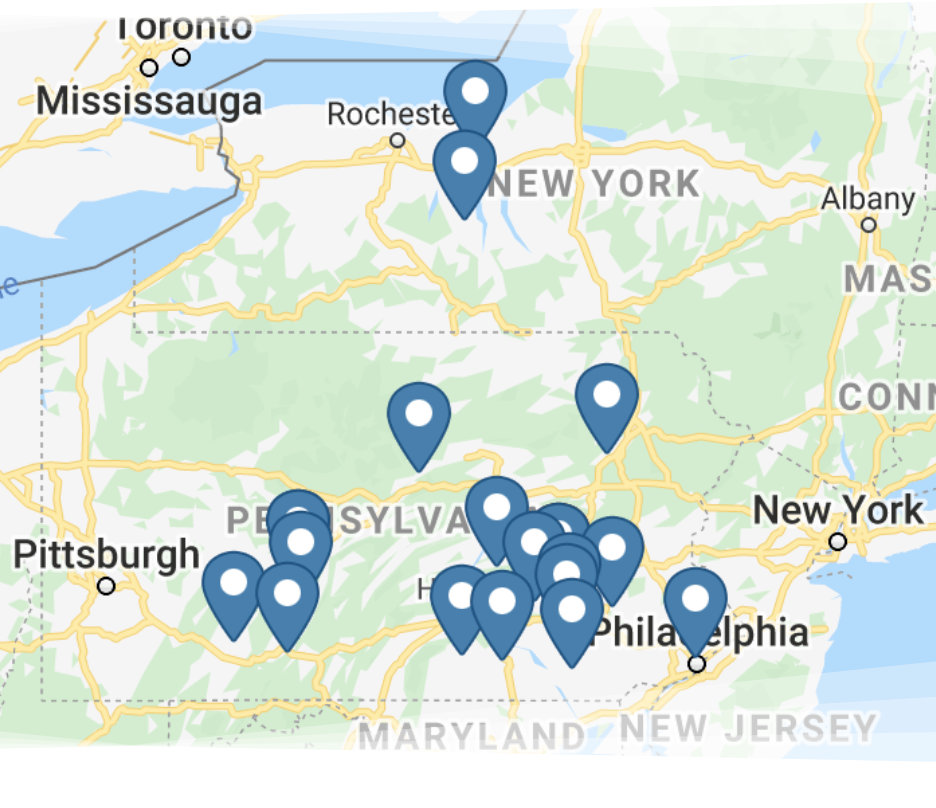

With more than 130 team members working at numerous Martin Water Conditioning locations, you can be sure we’re nearby so we can serve you better and faster.
Just give us a call to schedule service: (800) 887-7555
We service portions of Pennsylvania, New York, Maryland, Delaware and West Virginia.
“I want people to be aware of what’s in their homes, and how to improve and protect these things – like their water. I am excited to partner up with Kinetico to help homeowners understand the importance of having their water analyzed and diagnosed by water professionals and then lead them to Kinetico’s reliable and customized solutions to treat their water.”
Kinetico is a Proud Partner of Mike Holmes


Mostbet casino həyəcan və imkanlar dünyasına dalın, burada hər bir mərc əhəmiyyətli uduşlara səbəb ola bilər.
Highroller Casino VIP program offers enhanced rewards and personalized service for its most loyal players.
Do you know what is in your water?
Headquartered in Myerstown, Pennsylvania, Martin Water Conditioning has been satisfying customers for more than 40 years by installing and servicing water softeners, water filters, drinking water systems, and specialty water treatment systems that solve a variety of water problems.
Copyright 2023 Martin Water All rights reserved